
Morinaga Milk’s Probiotic Bifidobacterium longum subsp. longum BB536 Receives ANVISA Approval with Gut Health Functional Claim in Brazil
-
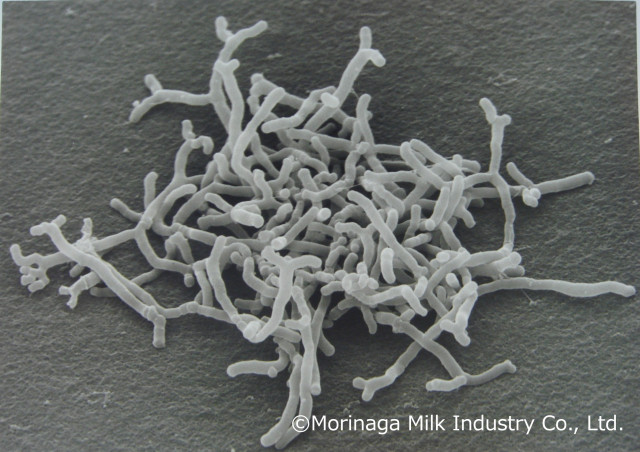
Bifidobacterium longum subsp. longum BB536, a clinically effective and well-established multifunctional probiotic strain, has a history of over 50 years of human use, supported by more than 240 scientific studies as of March 2023. Bifidobacterium longum BB536 strain has been recognized globally as a leading functional probiotic ingredient, holding Generally Recognized as Safe (GRAS) status in the U.S. for use in conventional foods and infant formula. It has also been approved as a “New Food Ingredient” in China for use in infant and toddler foods under the age of three. (Graphic: Business Wire)
TOKYO--(Business Wire / Korea Newswire)--Morinaga Milk Industry Co., Ltd. (TOKYO:2264), a leading Japanese dairy product company, is pleased to announce that its proprietary probiotic strain Bifidobacterium longum subsp. longum BB536 (hereafter referred to as Bifidobacterium longum BB536) has obtained approval from the Brazilian Health Regulatory Agency (Agência Nacional de Vigilância Sanitária, ANVISA). This approval, granted on July 27, 2023, allows for the utilization of Bifidobacterium longum BB536 in dietary supplements and conventional food products sold in Brazil. The strain has also been registered in ANVISA’s list of approved products.
Bifidobacterium longum BB536, a clinically effective and well-established multifunctional probiotic strain, has a history of over 50 years of human use, supported by more than 240 scientific studies as of March 2023. Bifidobacterium longum BB536 has been recognized globally as a leading functional probiotic ingredient, holding Generally Recognized as Safe (GRAS) status in the U.S. for use in conventional foods and infant formula. It has also been approved as a “New Food Ingredient” in China for use in infant and toddler foods under the age of three.
This recent approval by ANVISA allows Bifidobacterium longum BB536 to be used in dietary supplements and various conventional foods. At the same time, it is approved to claim that “Bifidobacterium longum BB536 may contribute to the health of the gastrointestinal tract.”
The approval holds significant implications for Morinaga Milk’s global growth strategy. As one of the objectives outlined in the company’s 10-year vision, Morinaga Milk aims to achieve an overseas sales ratio of at least 15% by the fiscal year ending March 31, 2029. This approval aligns with the company’s commitment to expanding its presence in international markets, including strengthening partnerships with major infant formula manufacturers and sales for dietary supplements all over the world.
The Brazilian probiotic supplement market is growing rapidly, with a compound annual growth rate of approximately 9% from 2013 to 2022, and the market size has more than doubled to approximately US$200 million[*1]. Morinaga Milk anticipates a promising future in the Brazilian market.
[*1] Source: Euromonitor International, August 2023
“We are thrilled to announce the approval of BB536 by ANVISA for use in supplements and conventional foods in Brazil,” stated Dr. Yoshihiko Ushida, General Manager of the International B to B Business Department of Morinaga Milk. “This achievement underscores our commitment to producing high-quality, scientifically validated probiotics that contribute to the well-being of consumers around the world.” As Morinaga Milk moves forward, the company is enthusiastic about the opportunities presented by this milestone.
About Morinaga
Morinaga Milk Industry Co., Ltd. is one of the leading dairy product companies in Japan with a century of history harnessing the nutritional properties of dairy products and functional ingredients. Morinaga Milk is also a key global probiotics manufacturer that excels in innovative technology and offers a premium line of probiotics and functional ingredients worldwide. Since the 1960s, Morinaga Milk has been engaged in research on the safety, functional health benefits, and mechanisms of action of probiotic bifidobacteria to better understand their role in maintaining human health. For more information about Morinaga Human-Residential Bifidobacteria (HRB) probiotics, please visit us at https://morinagamilk-ingredients.com/.
View source version on businesswire.com: https://www.businesswire.com/news/home/20230907986781/en/
Website: http://www.morinagamilk.co.jp/english
View Korean version of this release
Contact
Morinaga Milk Industry Co., Ltd.
Investor & Public Relations Department
Mitsunori Watanabe / Kazuaki Kajikawa
International Division
Junichi Minami / Chyn Boon Wong
This is a news release distributed by Korea Newswire on behalf of this company.

